Overview
Protocol Design
Engage with our team of Biostatisticians from the earliest stages of study development.
More Information
Sample Size
We have expertise and software necessary to perform sample size calculations for any design.
More Information
SAP Development
We will prepare all statistical methods necessary to meet the needs of the study.
More Information
IDMC Support
We have extensive experience and expertise providing quality independent statistical support and customized reports and graphs.
More Information
CDISC Datasets
Our knowledgeable and experienced team can upgrade your database to be SDTM and ADaM compliant.
More Information
Statistical Analysis
We have the ability and experience to perform all necessary statistical analyses over the course of the study.
More Information
Study Reporting
Work with us and ensure that all study reporting is accurate, thorough, and timely.
More Information
Regulatory Support
Our team has experience compiling quality submissions for regulatory agencies.
More Information
Publishing Results
Collaborate with us to share your study results with the Scientific community.
More Information

Customized Statistical Reporting
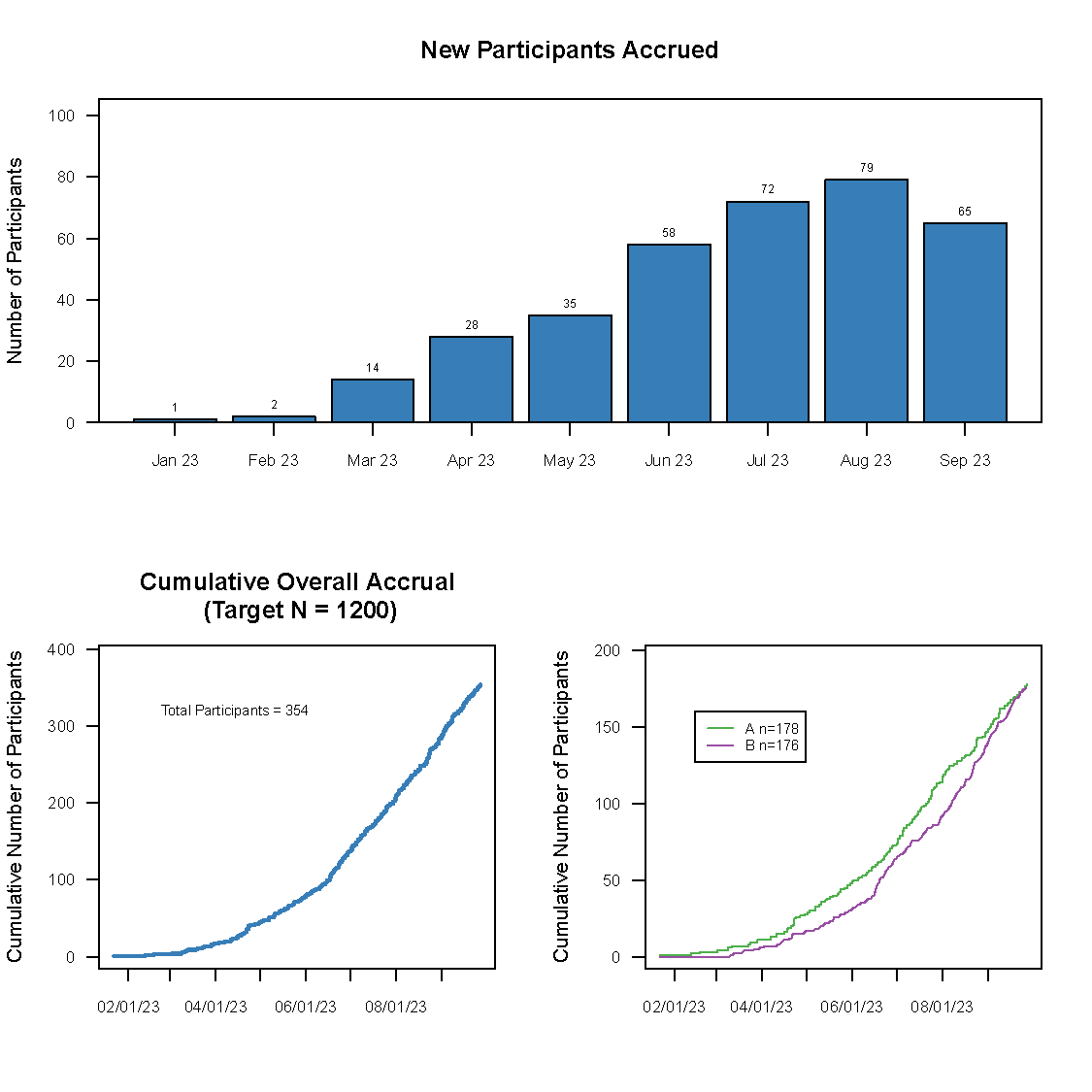
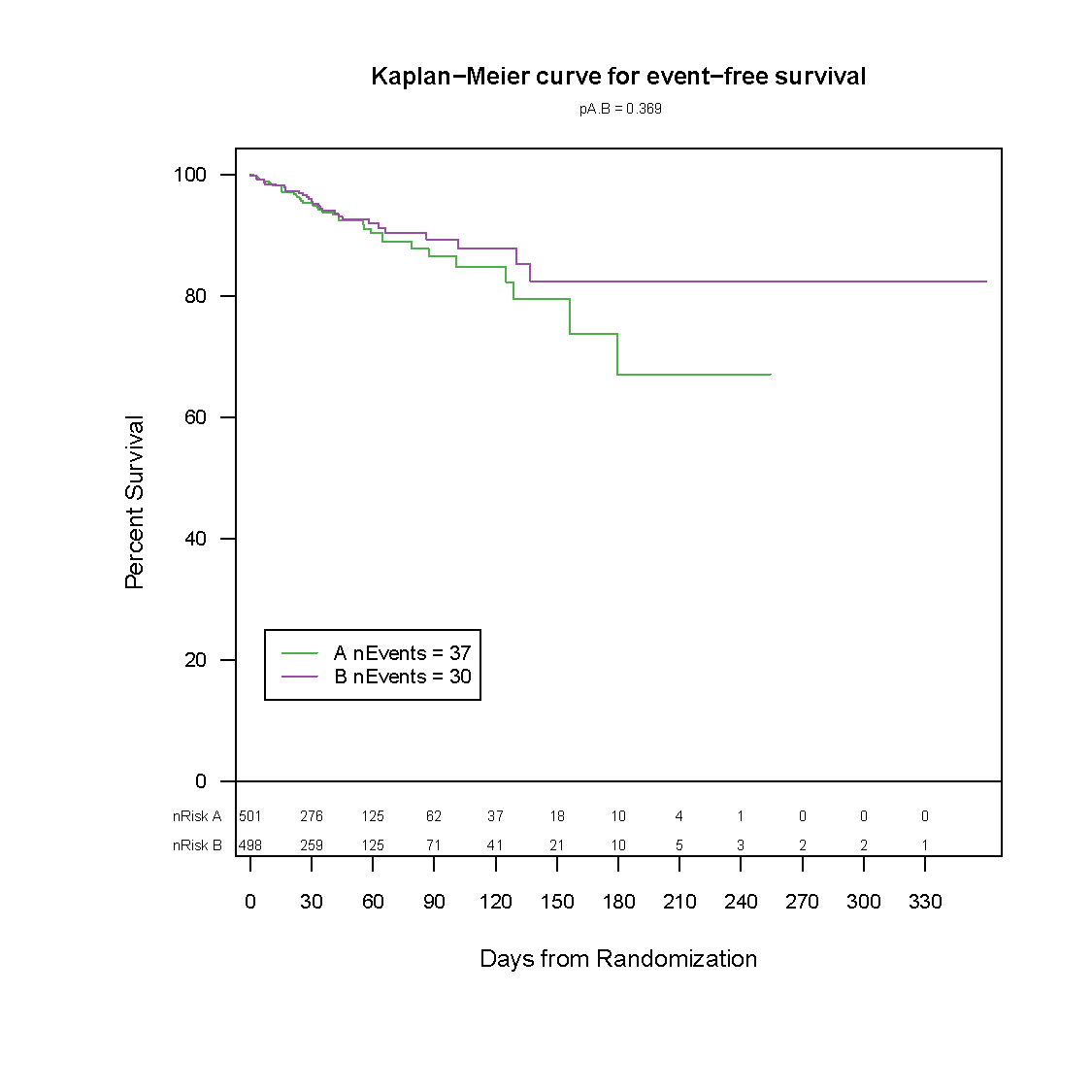
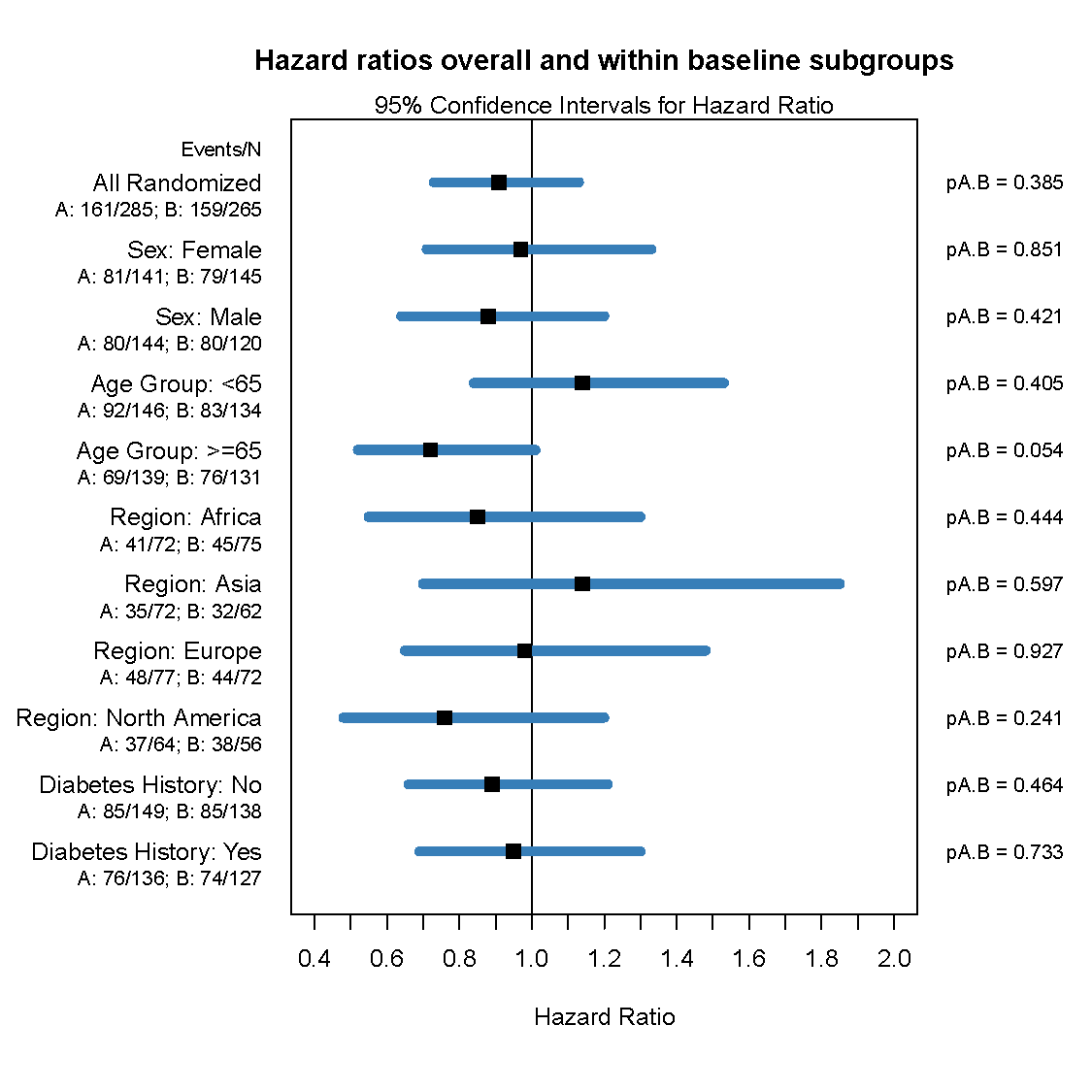
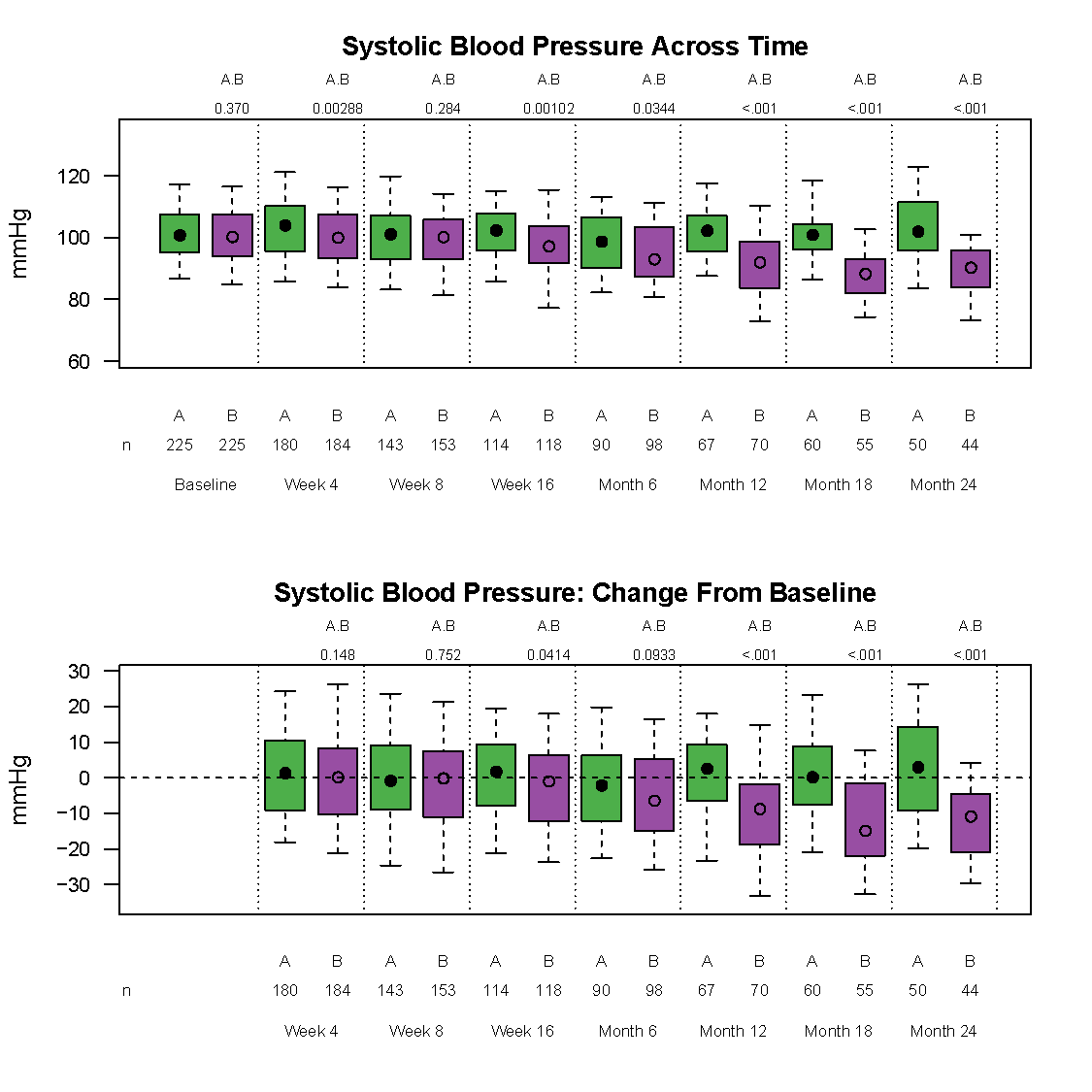
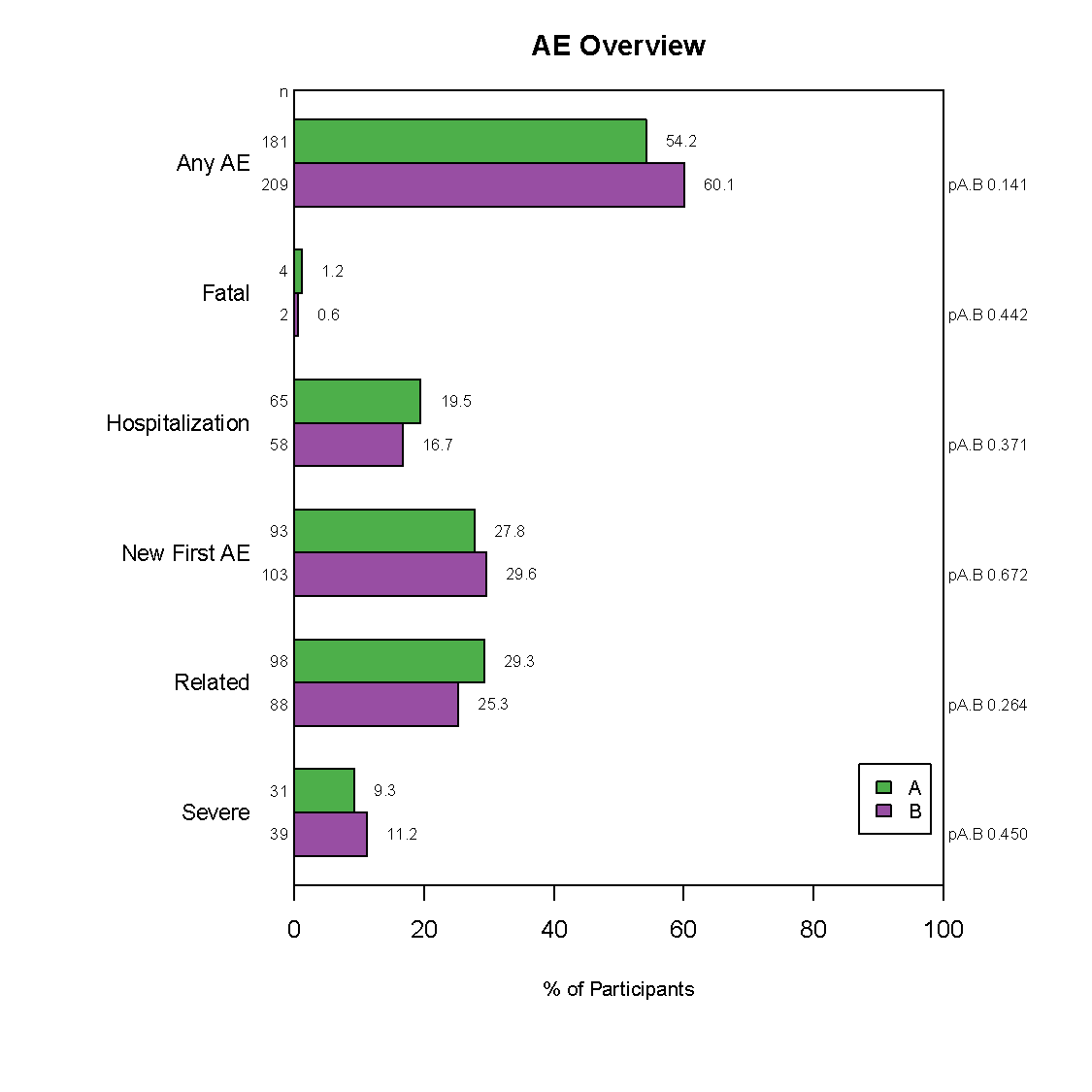
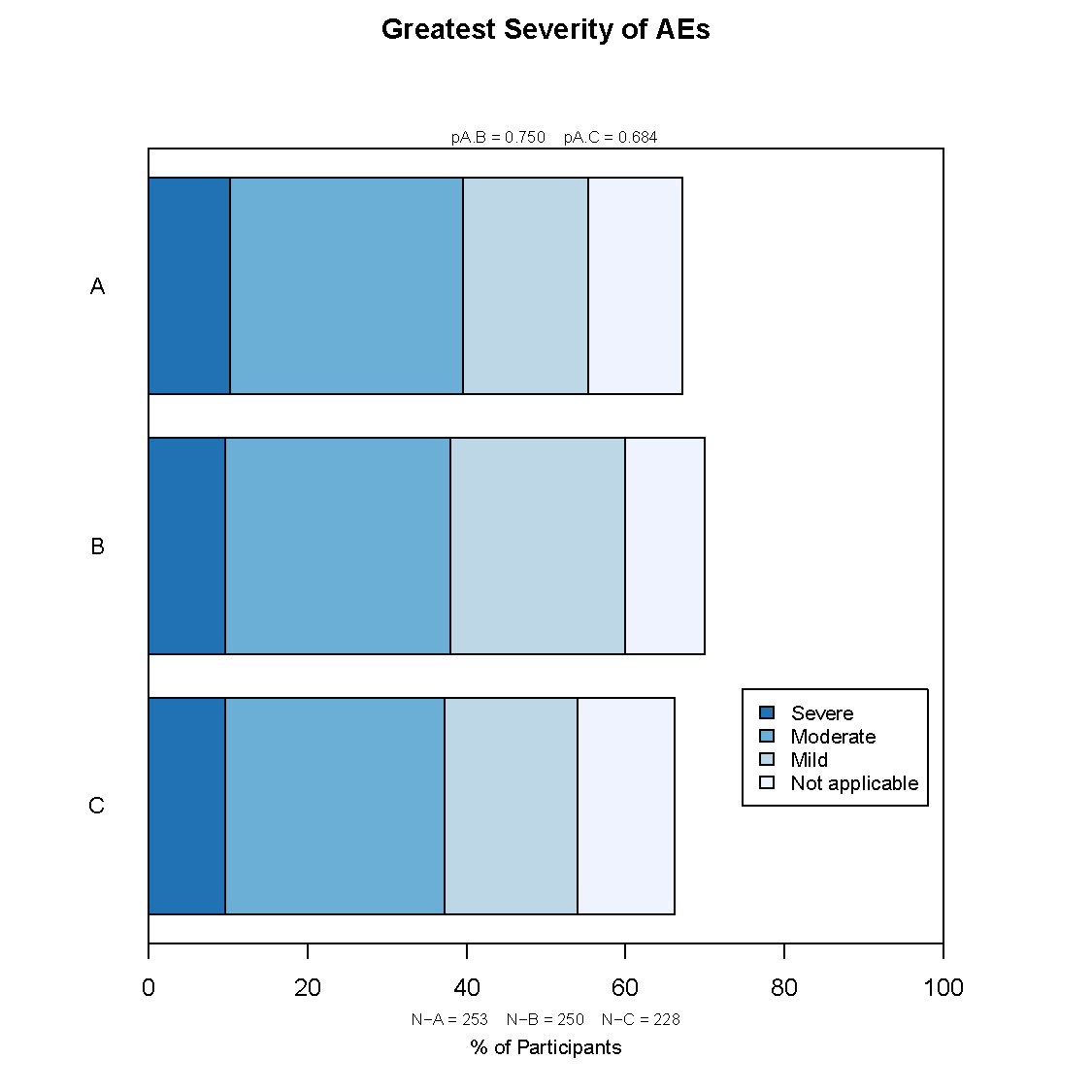
Frontier has developed a graphically based reporting system which can be utilized to efficiently and effectively display study results over the course of a study. This unique reporting style sets us apart as it enables reviewers to quickly zero in on the information they need.
Expertise
Our broad experience with all aspects of clinical trials enables us to understand and anticipate the specific needs of each study. Beyond producing reports, our Statistical team engages scientifically with the studies at hand and provides intellectual input.
Teamwork
Frontier assigns a team of biostatisticians and programmers to fit the needs of each study. In addition, our thorough processes include support for validation of all outputs, as well as support from our Quality Assurance department to ensure all compliance of all our work.
Reporting
Our reporting style is flexible to best address the needs of all collaborators. In particular, our highly graphical approach to Independent data Monitoring Committee (IDMC) reports hone in on what the IDMCs are looking for. This enables the the IDMC to review a lot of material in a relatively efficient way.
Security
Frontier takes great care to protect the integrity and confidentiality of trial data, including working in a computing environment that meets key regulatory security standards such as HIPAA Security Rule and FISMA.
Continuity
The statistical team work closely with all collaborators throughout the entire duration of the trial, from inception through closeout. Many statisticians have worked at our organization for a long time, and this unique level of continuity is beneficial to all trials, especially long term ones.
Responsiveness
Our statistical team ensures that a snapshot of the study data is readily accessible, as needed, to quickly and efficiently address all requests as the study progresses.

Our Statistical Team
Our statistical team has decades of experience in providing guidance on the optimal statistical and study design needed to address the scientific hypothesis, and works closely with the data management team to assist in case report form development, randomization guidelines, and monitoring plans.